BASIC KNOWLEDGE Light Emitting Diode (LED): Definition, types, and more
Related Vendors
A light emitting diode (LED) is one of the most widely used semiconductor devices. Made from compound semiconductors, LEDs efficiently convert electrical energy into light. This article outlines their construction, working principle, operation, and main types in detail.

LED was originally an idea of English engineer H. J. Round. Many researchers developed the initial LED diodes but they showed less progress. In 1962, American engineer Nick Holonyak from General Electric Company invented the most important device in LED history. The fully functional LED made from GaAsP (Gallium Arsenide Phosphide) emitted visible light. After the event, LED history never stumbled upon scaling. Until now, LED lights have proven to be efficient and cost-effective day by day.
Definition LED diode
An LED diode or Light Emitting Diode is a two-terminal semiconductor device that glows upon the application of forward voltage. Doped compound semiconductors construct LED diodes in a variety of colors. LED diodes work on the principle of electroluminescence to generate a monochromatic output.
LED diode symbol
The LED diode symbol resembles a conventional PN junction diode symbol.
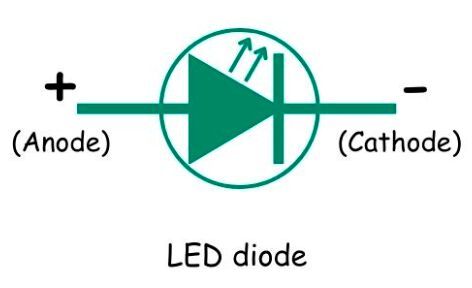
The circle encompassing the PN junction depicts the transparent plastic protective case of the LED diode. The outward arrows show photons (E = hv) as the output. However, the box is not shown in the LED symbol sometimes.
LED diode working principles
LED works on the principle of electroluminescence. The conversion of electrical energy into optical energy is called electroluminescence. When an electron jumps from a higher energy band to recombine with a hole in the lower band, radiation is released. The percentage of radiation can either be in the form of crystal lattice vibration, photon (E = hv), or a combination of both.
The choice of semiconductor and its band gap energy in eV determines the frequency and wavelength or the color of monochromatic light emitted through the LEDs. The nature of released light can be either infrared, visible, or ultraviolet.
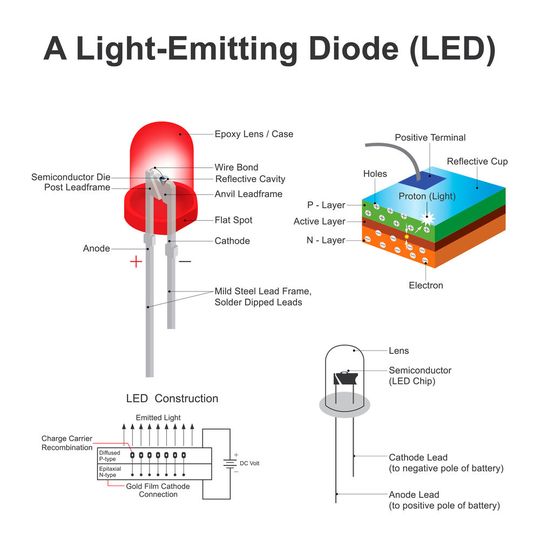
LED structure
Construction
In an LED diode, a P layer is diffused over the epitaxial N+ substrate. There is an active region engineered between the two layers. LEDs with P+ substrates are not common. Two electrodes are connected to the N+ substrate and P layer for electrical connection. The longer terminal of the LED diode is the anode (positive) and the shorter is the cathode (negative).
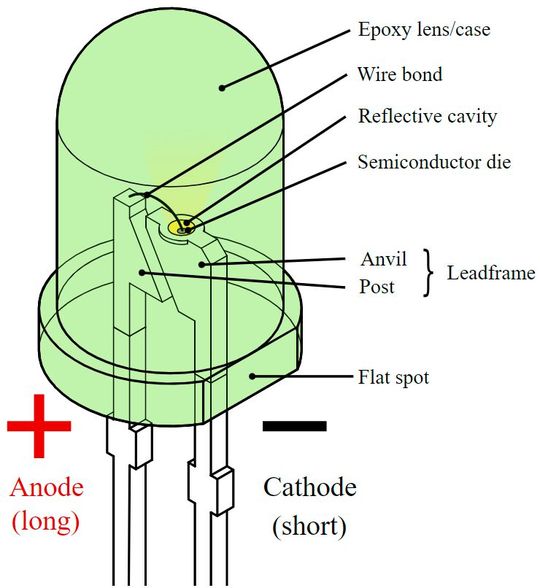
The LED diode is protected in a transparent plastic case. The shape of the plastic case is like a reverse chemical lab test tube. The top of the covering resembles a dome-like curvature. The idea of putting an LED in a protective plastic case is to ensure the safety of the semiconductor from damage and prevent total internal reflection.
Total internal reflection
The radiated photons can be absorbed within the structure. The semiconductors used in LED diodes have a high refractive index compared to air’s low refractive index. The light is emitted in the region forming a cone, known as the light cone. The angle of photons striking the air’s surface is larger than the critical angle. It causes total internal reflection that makes photons return back to the surface of the semiconductor. The process of total internal reflection functions like a hypothetical mirror.
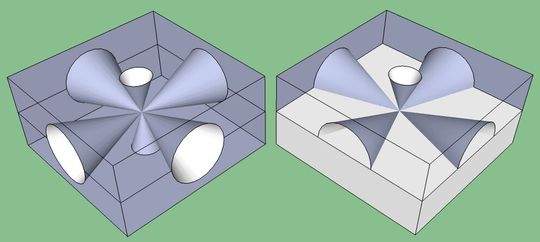
The phenomena cannot be seen directly through the eyes. The trapped light that fails to exit the LED releases in the form of heat. The solution to avoid internal reflection is to design the LED diode like a fresnel lens or microsphere and cover it with a plastic case. In the diagram, the reflective cavity in the LED is shown to prevent internal reflection and scatter the light. The covering widens the angle to prevent reflection. Such construction and protection redirect the photon beam in different directions for desirable results.
Quantum theory behind LED
Absorption
The process of absorption occurs when junctions are created in the diode. It is an initial process that determines the state of particles in an unbiased LED.
Suppose E1 and E2 are two energy levels for a semiconductor. E1 is the valence band and E2 is the conduction band. The energy of electrons in the valence band is lower than the electrons in the conduction band. In short, E1 is stable compared to E2. The absorption process starts when the electrons in the valence band absorb incoming energy in the form of photons or heat from any source.
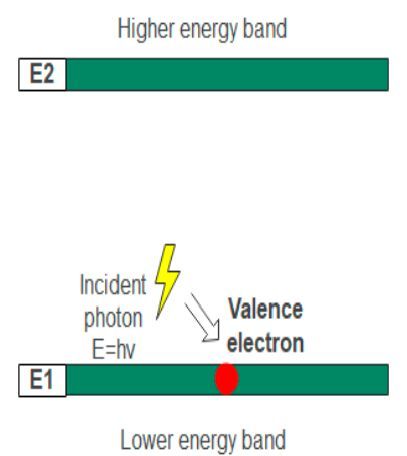
For LEDs, an incoming photon hits the surface of a semiconducting material. As a result, the semiconductor absorbs the photon. The electrons jump from the lower energy level valence band to the higher energy level conduction band. The process in which the energy from a source is absorbed to excite an electron from low energy to a higher energy band is called absorption.
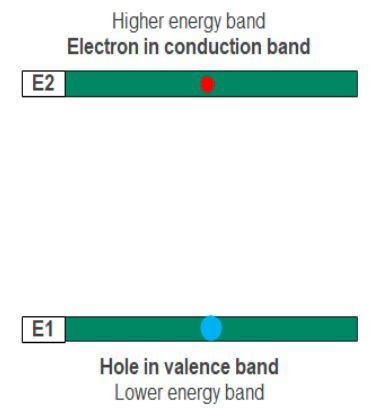
The electron leaves a gap in the valence band as it jumps to the conduction band. The gap functions as a positively charged particle called a hole. The absorption process places free electrons in the conduction band while holes lie in the valence band.
Spontaneous emission
In our laser diode article, spontaneous emission was stated to be the working principle behind LEDs. Modern LED diodes are faster and more efficient due to the working principle of electroluminescence. Spontaneous emission is a quantum process that occurs naturally in a semiconductor. As discussed above, the free electrons are in the conduction band while the holes reside in the valence band. The process of spontaneous emission initiates when electrons and holes recombine at the junction.
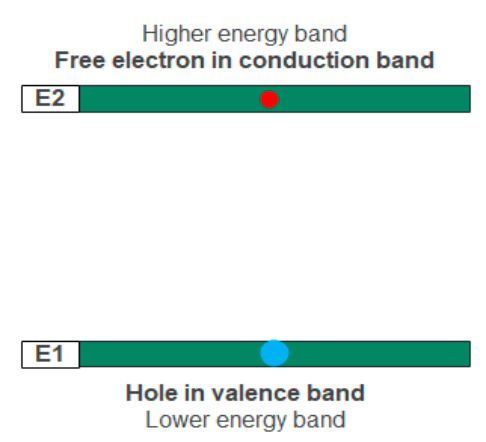
Electrons jump again but from the higher energy conduction band to recombine with holes in the lower energy valence band. The recombination process releases photons. In the diagram below, electrons in the E2 energy level jump to a lower E1 energy level for recombination. In theory, a single recombination event in an LED diode releases a photon.
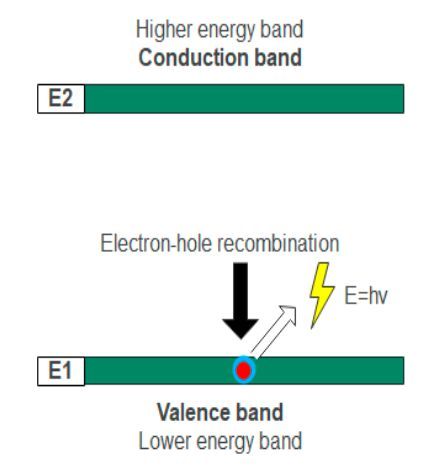
Spontaneous emission can be defined as the process where electrons jump from higher energy levels to recombine with holes in the lower energy levels while releasing photons in the output. In LEDs, the spontaneous emission process occurs “naturally”. Spontaneous emission should not be considered stimulated emission as both are different processes. The stimulated emission process is the working principle behind laser diodes instead.
Concept of Direct and Indirect Semiconductors
As per the above-mentioned theory, most compound semiconductors are suitable for making optical diodes like LED and laser diodes. This is because compound semiconductors are doped specifically to release photonic energy upon recombination.
Direct band gap semiconductors
Direct band gap semiconductors release a higher percentage of energy as photons instead of thermal radiation. The top of the valence band is called the valence band maximum (VBM) and the bottom of the conduction band is called the conduction band minimum (CBM).
In direct band semiconductors, VBM and CBM have the same momentum in k-space. The CBM lies just above the VBM for easy transition of electrons. In contrast, electrons can jump from the higher energy conduction band to the lower energy valence band without changing the momentum.
As there is no change in momentum, efficient transmission releases photons. The photonic energy corresponds to the small difference between the energy gap expressed in eV.
Indirect band gap semiconductors
Indirect band gap semiconductors dissipate heat in a higher percentage from energy conversion during recombination instead of photonic radiation. VBM and CBM have different values of momentum in k-space. The CBM does not directly exist above the VBM.
Electrons can jump from the higher energy conduction band to the lower energy valence band with an additional need to change the momentum. The change in moment triggers the release of a “phonon”. A phonon is a vibration of a crystal lattice. As the motion of particles releases heat, a phonon can be called thermal radiation.
However, indirect band gap semiconductors like doped gallium phosphide (GaP) and gallium arsenide phosphide (GaAsP) are exceptions known in the industry to emit photons.
:quality(80):fill(efefef,0)/p7i.vogel.de/wcms/5f/fe/5ffedb2e0ffa6/listing.jpg)
LED circuit diagram
The band gap energy of compound semiconductors is higher than that of silicon. It makes forward voltage drop between 1.5 V to 5 V. In simple words, a high value of voltage is required to turn on the LED. A high voltage generates a high current in the circuit.
A resistor must be used in the LED circuit to limit the flow of current and avoid damage. The resistor is connected to the circuit to maintain the glowing uniformity of the LED diode. The value of the resistor depends on the supply voltage and type of LED semiconductor diode. One can check resistor color codes to select suitable resistors.
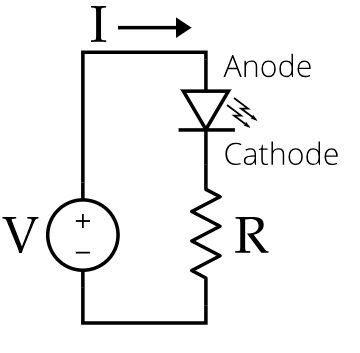
LED diode operation (electroluminescence)
The process of spontaneous emission occurs naturally in the LED. However, it is not sufficient to generate a bright and stable output. Electroluminescence is the main working principle behind LEDs. When voltage is applied to the LED, recombination takes place to release a photon. The process in which electric current interacts with the semiconductor or boosts radiative electron-hole recombination is called electroluminescence.
In comparison to spontaneous emission, electroluminescence and spontaneous emission are quite similar. Electroluminescence can be called a type of “artificial spontaneous emission” triggered through a voltage source.
LED: Zero bias
When there is no voltage supply to LEDs, no current flows through the LED diode. To understand LED functioning, let us return to diode theory from our basic knowledge diode article. Electrons are the majority charge carriers in the N-region and holes are the majority charge carriers in the P-region. Initially, the electrons in the N-side diffuse into the P-side, and holes in the P-side diffuse into the N-side.
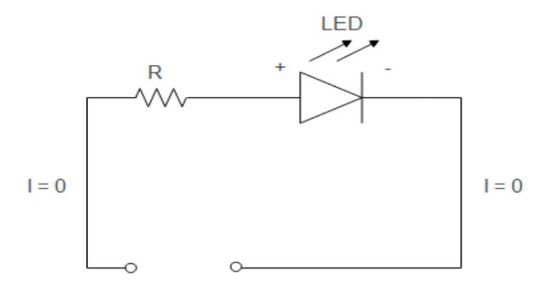
The diffusion of majority charge carriers in P and N regions results in the formation of an insulating layer inside the diode called the depletion layer or space charge region. The depletion region is devoid of charge carriers. It prohibits charge carriers from moving for current flow in the LED. The process of absorption explains the current state of LEDs into zero bias. The application of external bias changes the width of the depletion region to cause/block current flow.
LED: Forward bias
In order to initiate current flow, LEDs must be forward-biased. The p-side is connected to the positive terminal of the battery and the n-side is connected to the negative terminal of the battery. As explained above, electrons reside in the higher-level conduction band while holes reside in the lower energy valence band.
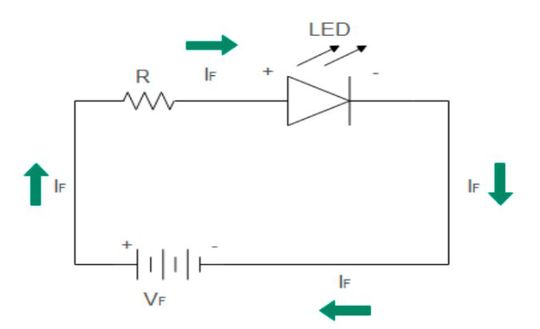
A strong electric field injects electrons and holes into the junction. The width of the depletion region decreases to support electron-hole recombination. Several recombination events of injected carriers release photons at the site of recombination. The process of electroluminescence releases photons as the output.
The current flows through the circuit and the LED continues to glow as long as it is in forward bias. The brightness of the LED diode increases only up to a saturation point, after which there is no change in brightness even with increasing forward current. The color of LED light- wavelength and frequency depends upon the type of semiconductor chosen for construction and their band gap.
LED: Reverse bias
The p-side is connected to the negative terminal of the battery and the n-side is connected to the positive terminal of the battery. The width of the depletion region increases to prohibit majority charge recombination. The minority charge carrier holes in the N-region and electrons in the P-region move through the LED to recombine. This small current in microamperes called reverse leakage current tends to release photons upon recombination. A practical example could be a slightly irregular glowing LED.
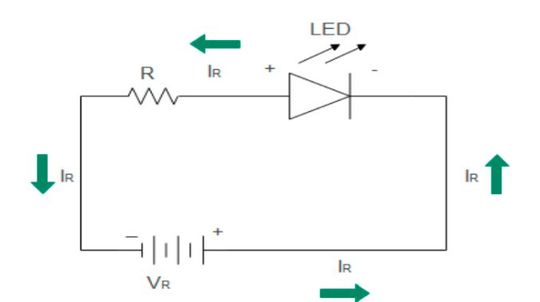
However, reverse bias operation is not desirable in LED diodes. A slight increase in reverse voltage (up to 5 V) causes a breakdown in the diode to damage the LED. Due to light doping of the LED, avalanche breakdown occurs during reverse bias. Reverse breakdown operation is an LED failure mode where the LED diode fails to operate. In commercially available LEDs, the value of reverse breakdown voltage is from 3 V to 5 V, much less than other types of diodes.
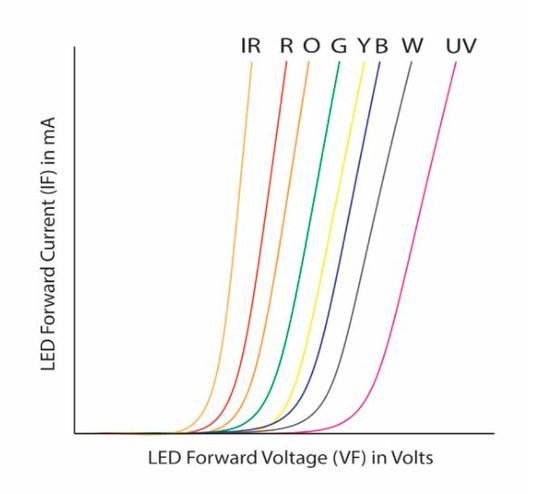
LED characteristic curve
The output characteristic curve of LED diodes is the graphical relationship between forward current and forward voltage.
The characteristic curve below shows the voltage-current relationship for different LED colors.
Types of LED diodes
Both LED and laser diodes are types of optical diodes. An optical diode converts electric current to light or vice versa. The section ahead lists and describes various types of diodes and their applications.
:quality(80)/p7i.vogel.de/wcms/ff/22/ff22163c256104c22639a24e8af3565b/0113258006.jpeg)
TYPES OF DIODES - OVERVIEW
The different diode types explained
Types of Light Emitting Diode
Depending upon the output light wavelength and frequency, there are three broad categories of LEDs. The output from Infrared and Ultraviolet LEDs is not visible to the human eye.
Infrared LED
LED diodes made from GaAs (Gallium Arsenide) and AlGaAs (Aluminium Gallium Arsenide) release photons in the infrared spectrum of light. The infrared LED diodes release infrared light with wavelengths greater than 760 nm. Contrary to popular belief, the prime function of LED diodes is not limited to glowing. Applications of infrared LED diodes include home automation, IoT, security systems, and opto-coupling.
Visible LED
The below-mentioned category of “lightning LED diodes” comes under visible LEDs. Unlike infrared and ultraviolet LEDs, output light is viable to the human eye. The sole purpose of these LEDs is to glow to confirm the flow of current. Each LED has a size in the range of 2 mm to 5 mm and offers a forward voltage drop of 1 V to 4 V.
Ultraviolet LED
An ultraviolet LED is sometimes called a violet or a purple LED. The ultraviolet LED diodes release light below the wavelength of 400 nm. Compound semiconductors like BN (Boron Nitride) and Aluminium Nitride (AlN) are used to make ultraviolet LED diodes. A report reveals that UV LEDs are environmentally safer than traditional LED diodes. Applications of UV LED include optical data storage, coatings, forensics, and detection.
Lightning LEDs
The classification under “lightning LEDs” is based on the color of light emitted. Each color LED uses a different type of compound semiconductor and has different properties. The lighting category can also be called “general purpose LEDs” or “visible range LEDs” (above-mentioned) as the primary function of this category is to generate visible light as an output. Lightning LED diodes come in separate DIP packages with low ratings.
Red LED
As red has the highest wavelength out of the visible spectrum, red LEDs release a strong beam that can penetrate layers. RED LEDs are used in a variety of medical applications. Compound semiconductors GaAsP (Gallium Arsenide Phosphide) are used in constructing RED LEDs.
Blue LED
Compound semiconductors GaN (Gallium Nitride) are used in constructing blue LEDs. Researchers are trying to build blue LEDs using SiC substrate. However, blue LEDs have a major disadvantage of having a high forward voltage drop of 5 V. The practical applications consume more power and increase implementation costs.
Green LED
According to the standard response curve of the human eye, the human eye responds to green more than any existing color. Compound semiconductor GaP (Gallium Phosphide) is used to manufacture green LEDs. An Aluminum dopant makes AlGaP to engineer the band gap and control the wavelength.
Yellow LED
Yellow or amber LEDs are among the common types of LED diodes used in electronic projects at schools, and colleges along with red and green. Compound semiconductors like AlGaInP (Aluminium Gallium Indium Phosphide) and GaAsP (Gallium Arsenide Phosphide) are used to make yellow LEDs.
Orange LED
Similar to yellow or amber LEDs, compound semiconductors like AlGaInP (Aluminium Gallium Indium Phosphide), GaAsP (Gallium Arsenide Phosphide), and GaP (Gallium(|||) Phosphide) are used to make yellow LEDs. Orange LEDs are used in decorative lighting for festivals and electronic device indicators.
Pink LED
As pink color is not a part of VIBGYOR, using a dye or combination of two LEDs can build a pink LED. A blue LED is coated with two layers of phosphor. Another way is to use a bi-color LED (two LEDs in one package) for pink light emission.
RGB LED
An RGB or tricolor LED consists of red, blue, and green colors in a single package. Modifications of different intensities through a controller can produce a variety of LED colors. RGB LED has three terminals for red, blue, and green with a common anode or cathode.
White LED
No semiconductor produces white light upon recombination. The white LED diode can be made by coating phosphor over different LEDs. The process is called phosphor conversion. A common way to manufacture white LED diodes is to coat blue LEDs made from GaN (Gallium Nitride) with YAG phosphor
Initially, a yellow light is emitted. The mixing of yellow and blue light generates white light in the output. Another method is to implement color mixing techniques in red, blue, and green LED diodes. White LEDs are used in commercial and residential lighting.
OLED
Organic LED, aka OLED, is a popular LED diode type of display. The word “organic” states that OLEDs use organic compounds to release light. Thin films of organic material are deposited over the semiconductor substrate. When electric current is applied to the device, the carbon-based layer causes millions of pixels to emit light.
OLEDs offer a high-quality output in terms of high contrast ratio, picture clarity, and color gamut (wide range of colors for a device). OLED display TVs are sold at high prices in the world of consumer electronics. The display of the OLED TV is of black color.
Perovskite LED
A perovskite LED (PeLED) is an emerging LED technology for display, lighting, and octo coupling. Perovskite is a special crystal structure in the formula of ABX3. Most perovskites are a mineral compound of calcium, titanium, and oxides CaTiO. Some perovskites are considered to be the future of solar cells as well.
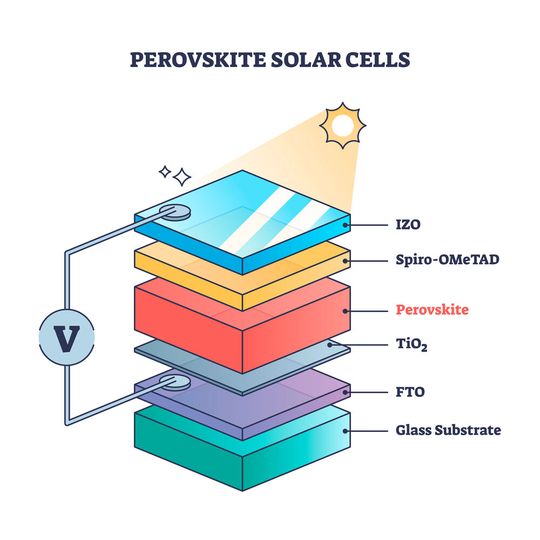
Researchers describe a compound of perovskite, lead, and halogen to make LEDs. Theoretically, PeLED diodes are assumed to be a thousand times more efficient than OLEDs and cheaper to manufacture. A PeLED uses perovskite as a layer to emit photons with high precision. As of 2024, PeLEDs are not commercially available.
Quantum-dot LED
A quantum-dot LED incorporates “quantum dots” to emit monochromatic light. Quantum dots (QD) are extremely small semiconductor crystals in the order of 1 to 10 nm. A separate layer functions in the LED structure to excite quantum dots for releasing light.
The basic principles of quantum mechanics in QD LEDs offer high precision, efficiency, saturation, contrast ratio, and better control over the emission. QD LEDs can be engineered to produce output in infrared, visible, and UV range. Possible applications of QD LED are QDD (Quantum-Dot Displays) for augmented and virtual reality, watches, and vehicles.
High-power LED
High-power LED diodes are used in various power electronic applications like huge headlights for streets, high-power lamps, underwater lighting, and industrial equipment. High-power LEDs have a high efficacy.
Efficacy can be defined as the ratio of total luminous flux in lumens and input electrical power in watts. Simply put, efficacy is the measure of electroluminescence or the conversion of electrical power into optical power. Just like other power electronic devices, high-power LED diodes can overheat. A heat sink or a cooling material is required to protect these high-power diodes from damage.
LED diode applications
LED diodes have found widespread applications across various industries and technologies, serving as versatile components in electronic devices and lighting solutions.
- LED diodes are used in electronic projects to detect flow of current. A glowing LED confirms that the current flows through the circuit.
- LEDs are popularly used in LCD displays (Liquid Crystal Displays) of calculators, watches, TVs, etc.
- LED lights are available in the form of bulbs, tube lights, flashlights, and lamps, for personal, residential, commercial, and outdoor lighting purposes. The traffic lights use red, yellow, and green LEDs.
- Automobile lightning through indicators.
- Alphanumeric LEDs display numbers on a screen. There are seven-segment, fourteen-segment, and sixteen-segment displays available. The working principle behind segment displays is to energize LEDs to show numbers and alphabets. Most alphanumeric LEDs have either a common anode or cathode. Initially, the segment displays were sold in DIP packages.
- Other applications: data communication, optical fiber, optocouplers, remote controls, detection, medical equipment, tattoo making, and many more.
LED diode advantages
LED diodes offer several key benefits that have made them a preferred lighting choice in many applications.
- The average lifetime of an LED is 25,000 hours or 2.8 calendar years.
- LEDs can emit light of intended colors.
- LEDs are smaller in size from 2 mm to 5 mm.
- LED diodes consume low power.
- LED diodes are shock-resistant.
- LED diodes are budget-friendly.
- Easy availability.
- Effective integration of smart lighting systems.
LED diode disadvantages
LED diodes, while offering numerous advantages, present a set of challenges that limit their performance and applications.
- The nature of the output beam is non-coherent and has a broad spectral profile.
- Slower than laser diodes.
- Susceptible to optical pollution.
- Flickering and dimming.
- Efficiency reduces in LED diodes when the current in the circuit increases.
- Temperature dependence.
- Quick failure due to overheating.
(ID:50125917)



:quality(80)/p7i.vogel.de/wcms/f5/d2/f5d2bce7c01775fe62d4c6ecebc8c5ba/0129188745v2.jpeg)
:quality(80)/p7i.vogel.de/wcms/cb/38/cb38bf951c0af8a8de423fedce2489d8/0129352475v2.jpeg)
:quality(80)/p7i.vogel.de/wcms/b8/13/b8139abf82c98aa04248a4a119d28c13/0129194616v2.jpeg)
:quality(80)/p7i.vogel.de/wcms/62/80/6280da00d591873bf8f0b2a70e5986e0/0129064771v4.jpeg)
:quality(80)/p7i.vogel.de/wcms/60/40/6040b2e00aef20b4f9d92e8ac9f79c32/0129349725v2.jpeg)
:quality(80)/p7i.vogel.de/wcms/b3/13/b313941dbf7adc57c6d144966106d82b/0129219607v2.jpeg)
:quality(80)/p7i.vogel.de/wcms/56/a4/56a4d9b6ee131a7a00b8b89dabf108f9/0128979281v2.jpeg)
:quality(80)/p7i.vogel.de/wcms/67/62/676279913d77e1db48eb5cbe9be4c767/0128937895v2.jpeg)
:quality(80)/p7i.vogel.de/wcms/f0/d8/f0d82f06ed1b7abb3245dfc4c317cb55/0127949994v2.jpeg)
:quality(80)/p7i.vogel.de/wcms/6e/e5/6ee5ad1dc45fd69a5a5718147605850a/0129347492v2.jpeg)
:quality(80)/p7i.vogel.de/wcms/7d/40/7d406bd959b3a9127c33a66157f9030a/0128339184v4.jpeg)
:quality(80)/p7i.vogel.de/wcms/65/22/65223bc58811ced76adbfa7b5615d532/0129061536v2.jpeg)
:quality(80)/p7i.vogel.de/wcms/23/ee/23ee4a97790d6009dbfd7d9577ffa723/0129220424v2.jpeg)
:quality(80)/p7i.vogel.de/wcms/3c/d1/3cd1cacbceb792ba63727199c61ca434/0127801860v2.jpeg)
:quality(80)/p7i.vogel.de/wcms/5a/a0/5aa0436498af618297961fd54ab36cdf/0126290792v2.jpeg)
:quality(80)/p7i.vogel.de/wcms/cb/30/cb30ebdca7fcaea281749cb396654eb3/0124716339v2.jpeg)
:fill(fff,0)/p7i.vogel.de/companies/5f/71/5f71d5f92a5f6/2000px-rogers-corporation-logo-svg.png)
:fill(fff,0)/p7i.vogel.de/companies/62/95/6295c25c8dc1a/schunk-sonosystems-300dpi.png)
:fill(fff,0)/p7i.vogel.de/companies/66/8b/668becd1c07eb/dowa-logo-word--1-.jpeg)
:quality(80)/p7i.vogel.de/wcms/7b/2c/7b2c4ddd6c8fd3b44fe8afc18f0d7e40/0115676667.jpeg)
:quality(80)/p7i.vogel.de/wcms/35/f2/35f243dd4bd4d57587bd7e4f7a85d6fa/0117752002v2.jpeg)