INTERNAL RESISTANCE How internal resistance affects battery performance
Related Vendors
Internal resistance in batteries reduces efficiency and lifespan by causing voltage drops and heat generation. It is influenced by factors like temperature, charge cycles, and manufacturing quality.
Internal resistance plays a significant role in battery performance, affecting efficiency, power output, and lifespan. In lithium-ion batteries, it influences how effectively energy is delivered. Power engineers should seek to understand internal resistance to help optimize the performance of power applications and better predict battery health over time.
What is internal resistance?
Internal resistance refers to the resistance within a power source, such as a battery or generator. In other words, it’s the name given to a property of components where their primary function is not to act as a resistor but does so anyway.
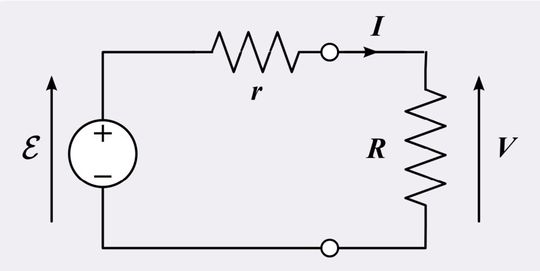
Internal resistance refers to the resistance within a power source. In the context of batteries, internal resistance is the opposition to current flow within it. Internal resistance can be modeled as a resistor in series with an ideal voltage source. The internal resistance (Ri) can be calculated using the formula:
Ri = (VOC - VL) / IL
where VOC is the open-circuit voltage, VL is the voltage under load, and IL is the current under load. As current flows through the battery, internal resistance causes a voltage drop, reducing the effective voltage available to the load. This leads to power loss, reduced efficiency, and increased heat generation.
Measuring internal resistance in batteries
Internal resistance can be measured using AC and DC methods. Electrochemical Impedance Spectroscopy (EIS) is one of the most accurate AC methods. It involves applying a range of AC signals at different frequencies and analyzing the battery’s response to calculate impedance. The AC injection method is another accurate approach that involves injecting a small AC signal, typically in the MHz range, into the battery and measuring the voltage response. This method is less intrusive and provides high accuracy.
DC methods are simpler but generally less precise. The pulse current method involves applying a brief high-current pulse to the battery, measuring the voltage change before and after the pulse, and calculating internal resistance using Ohm’s law. Load testing is another DC method where a low-value resistor is connected across the battery terminals, and the voltage drop is measured to determine resistance.
For accurate measurements, it is important to maintain proper connections between the battery and measurement equipment. Lithium-ion batteries should be measured at voltages between 3.6V and 3.8V. Temperature and state of charge must be controlled, and appropriate current and pulse time should be used for DC methods. In industrial settings, AC methods, particularly EIS, are preferred due to their accuracy and detailed analysis capabilities.
What contributes to internal resistance in batteries?
Several factors contribute to increased internal resistance. Battery chemistry plays a role, as different chemistries have varying resistance characteristics. State of charge affects internal resistance, which typically increases at very low and very high charge levels. Temperature is another factor, with lower temperatures increasing resistance and higher temperatures accelerating aging.
Age and cycling also impact resistance. As batteries undergo charge-discharge cycles, electrode degradation and electrolyte decomposition cause resistance to rise. Manufacturing quality affects resistance through electrode alignment, electrolyte distribution, and contact resistance. Battery structure, including electrode design and separator characteristics, plays a role in determining internal resistance.
:quality(80):fill(efefef,0)/p7i.vogel.de/wcms/62/0c/620cfcbf6f486/titelbild-webkon-pb-2022-03-15.jpeg)
WEB CONFERENCE: ENERGY STORAGE
Keys to the design and operation of battery storage systems
Usage conditions influence resistance over time. Depth of discharge and charging cut-off voltage can lead to changes in internal resistance. High-current demands increase polarization resistance, reducing efficiency. Electrolyte issues, such as insufficient electrolyte filling or changes in concentration, contribute to resistance growth. Manufacturing factors, including electrode coating density and rolling thickness, also play a role. Physical changes, such as corrosion, plate sulphation, and active material shedding, further increase resistance.
Internal resistance and battery lifespans
Internal resistance directly affects the lifespan of lithium-ion batteries by accelerating degradation and reducing overall performance over time. Heat generation is a major issue, as higher resistance leads to more heat during charge and discharge cycles. This excess heat speeds up chemical reactions, contributing to faster aging and, in extreme cases, thermal runaway.
As internal resistance increases with age, the battery's usable capacity decreases. Energy is lost as heat, and voltage drop under load, reducing runtime between charges. Voltage sag becomes more pronounced, leading to premature shutdowns or reduced device performance. Efficiency decreases as more energy is lost as heat rather than being available for useful work, accelerating component degradation.
Aging processes contribute to rising resistance, including electrolyte decomposition, electrode degradation, and current collector corrosion. This creates a feedback loop, further accelerating aging. Cycling the battery, especially in high-current applications, speeds up resistance growth, shortening its lifespan.
Engineers should consider these factors when designing batteries so that they can optimise performance and predict battery health and lifespan while keeping in mind that managing these factors can extend lifespan in high-power applications.
(ID:50294998)



:quality(80)/p7i.vogel.de/wcms/71/fd/71fdcc22d9a9bd2f42985f692c4aefa2/0128924236v2.jpeg)
:quality(80)/p7i.vogel.de/wcms/94/54/94548eaecd020681e558d563bc48ba1d/0128926221v2.jpeg)
:quality(80)/p7i.vogel.de/wcms/29/99/2999bb9af245dd31f4c837c1d9359046/0128923137v2.jpeg)
:quality(80)/p7i.vogel.de/wcms/10/78/107856328ef320cc081bf88e0baf95e8/0128685487v2.jpeg)
:quality(80)/p7i.vogel.de/wcms/67/62/676279913d77e1db48eb5cbe9be4c767/0128937895v2.jpeg)
:quality(80)/p7i.vogel.de/wcms/0f/a2/0fa2b5bdc21e408fd73e637d226d5210/0128681532v2.jpeg)
:quality(80)/p7i.vogel.de/wcms/4f/6f/4f6faf0ca6f748a2967d6b5bba7c88e1/0128682406v2.jpeg)
:quality(80)/p7i.vogel.de/wcms/ad/52/ad52f7b5542eff15ba54ec354d31b50d/0128681536v4.jpeg)
:quality(80)/p7i.vogel.de/wcms/1e/9c/1e9c45d6fcf2fb48dc47756e4cb20174/0128931043v2.jpeg)
:quality(80)/p7i.vogel.de/wcms/8b/42/8b4271e1bedea432ab03c83959e30431/0128818204v2.jpeg)
:quality(80)/p7i.vogel.de/wcms/87/5a/875a8fa395c1eec9677e075fae7f5e8e/0128793884v2.jpeg)
:quality(80)/p7i.vogel.de/wcms/2f/93/2f9364112e8c6ff38c26f9ba34d0f692/0128791306v2.jpeg)
:quality(80)/p7i.vogel.de/wcms/3c/d1/3cd1cacbceb792ba63727199c61ca434/0127801860v2.jpeg)
:quality(80)/p7i.vogel.de/wcms/5a/a0/5aa0436498af618297961fd54ab36cdf/0126290792v2.jpeg)
:quality(80)/p7i.vogel.de/wcms/cb/30/cb30ebdca7fcaea281749cb396654eb3/0124716339v2.jpeg)
:quality(80)/p7i.vogel.de/wcms/0b/b4/0bb4cdfa862043eac04c6a195e59b3e0/0124131782v2.jpeg)
:fill(fff,0)/p7i.vogel.de/companies/60/7e/607ec89d5d9b5/white-frame.jpg)
:fill(fff,0)/p7i.vogel.de/companies/62/a0/62a0a0de7d56a/aic-europe-logo.jpeg)
:fill(fff,0)/p7i.vogel.de/companies/5f/71/5f71d5f92a5f6/2000px-rogers-corporation-logo-svg.png)
:quality(80)/p7i.vogel.de/wcms/9a/af/9aafa5ad3d3d900a6e22adf6d06a9bf5/0126877798v2.jpeg)
:quality(80)/p7i.vogel.de/wcms/bf/a2/bfa277a5c1330e67e3ba50d9da823970/0127853966v2.jpeg)